What is the equilibrium equation for the following reaction?
FeS(s) + 2 H3O+ (aq) ⇌ Fe2+(aq) + H2S (aq) + 2 H2O (l)
A) Kc = 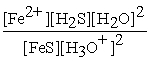
B) Kc = 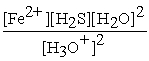
C) Kc = 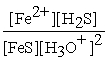
D) Kc = 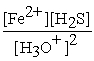
Correct Answer:
Verified
Q8: The oxidation of sulfur dioxide by oxygen
Q9: Which one of the following statements does
Q22: What is the value for Kc for
Q22: The decomposition of ammonia is: 2 NH3(g)=
Q23: If Kc = 2.0 x 1033 at
Q24: What is the equilibrium equation for the
Q30: What is the equilibrium equation for the
Q32: What is the equilibrium constant,Kc,for the reaction:
Q37: Given the reaction at a certain temperature:
Q40: The decomposition of ammonia is: 2 NH3(g)⇌
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents