Picture (1) represents the equilibrium mixture for the gas-phase reaction A + B ⇌ 2 AB at 298 K.If the volume of the equilibrium mixture is decreased,which picture (2) -(4) represents the equilibrium at the reduced volume? 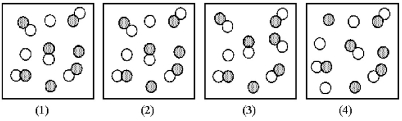
A) picture (2)
B) picture (3)
C) picture (4)
D) None of these
Correct Answer:
Verified
Q109: Picture (1)represents an equilibrium mixture of solid
Q114: Picture (1)represents an equilibrium mixture of solid
Q133: The equilibrium constant,Kp,equals 3.40 at 25°C for
Q134: Cyclohexane (C6H12)undergoes a molecular rearrangement in the
Q135: For the isomerization reaction:
Butane ⇌ isobutane
Kp equals
Q137: An equilibrium mixture of CO,O2 and CO2
Q139: The equilibrium constant is equal to 5.00
Q141: Kc is 1.67 × 1020 at 25°C
Q149: For the reaction CaCO3(s)⇌ CaO(s)+ O2(g)the equilibrium
Q156: A reaction in which reactants form products
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents