What is the equation relating the equilibrium constant Kn for the neutralization of a weak acid with a weak base to the Ka of the acid,the Kb of the base and Kw?
A) Kn = KaKbKw
B) Kn = 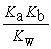
C) Kn = 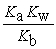
D) Kn = 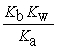
Correct Answer:
Verified
Q2: What is the pH of a solution
Q3: What is the approximate value of the
Q4: Which is a net ionic equation for
Q6: Which of these neutralization reactions has a
Q8: What is the approximate value of the
Q8: What is the common ion in a
Q9: What is the approximate value of the
Q11: When 50 mL of 0.10 M NH4Cl
Q12: The neutralization constant Kn for the neutralization
Q18: What is the pH of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents