What is the molar solubility of CaF2 in 0.10 M NaF solution at 25°C? The Ksp for CaF2 is 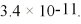
A) 8) 5 × 10-10 M
B) 3) 4 × 10-10 M
C) 3) 4 × 10-9 M
D) 2) 0 × 10-4 M
Correct Answer:
Verified
Q41: What is the molar solubility of AgCl
Q54: Calculate the solubility (in g/L)of silver carbonate
Q55: Calculate the Ksp for silver sulfate if
Q57: Which of the following metal hydroxides are
Q59: What is the molar solubility of lead(II)chromate
Q63: The following pictures represent solutions that contain
Q68: The following pictures represent solutions that contain
Q72: What is the molar solubility of AgCl
Q74: The balanced equation for the solubility equilibrium
Q75: What is the most soluble salt of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents