Calculate ΔS° for the following reaction.
N2(g) + 2 O2(g) → 2 NO2(g) 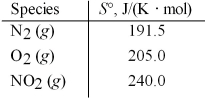
A) -156.5 J/K
B) -121.5 J/K
C) 15.5 J/K
D) 636.5 J/K
Correct Answer:
Verified
Q6: Assume a heteronuclear diatomic molecule,AB,forms a one-dimensional
Q19: The Boltzmann formula is S = k
Q21: For the process CaCO3(calcite)→ CaCO3(aragonite)ΔH° = -0.21
Q33: Calculate ΔS° for the formation of one
Q35: Which substance has the highest standard molar
Q36: Which has the highest entropy in each
Q37: Which has the highest standard molar entropy
Q40: Which has the highest standard molar entropy
Q40: During perspiration,
A)the entropy of the water evaporated
Q41: Other than only PV work,what reaction conditions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents