Which of the following are unstable with respect to their constituent elements at 25°C? 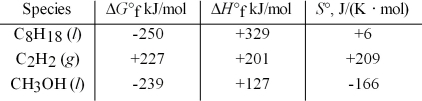
A) C8H18(l) ,CH3OH(l)
B) C8H18(l) ,C2H2(g)
C) C2H2(g)
D) CH3OH(l)
Correct Answer:
Verified
Q44: For the reaction below ΔG° = +33.0
Q49: Which of the following is true?
A)As a
Q52: A reaction has ΔH° = 61.9 kJ/mol
Q56: At 2600 K,ΔG° = 775 kJ for
Q58: Which is the lowest at 25°C?
A)ΔG°f for
Q68: At 25°C,?G°f is -620 kJ/mol for
Q72: Calculate the standard free energy change at
Q73: Which of the following is zero at
Q75: Calculate the standard free energy for the
Q139: In general,as a reaction goes to equilibrium
A)ΔG
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents