The figure represents the spontaneous deposition of iodine in which iodine vapor,I2(g) ,becomes crystalline iodine solid I2(s) : I2(g) : → I2(s) .What are the signs (+ or -) of ΔH,ΔS,and ΔG for this process? 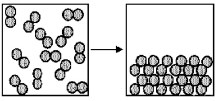
A) ΔH = +,ΔS = +,ΔG = +
B) ΔH = +,ΔS = +,ΔG = -
C) ΔH = -,ΔS = -,ΔG = +
D) ΔH = -,ΔS = -,ΔG = -
Correct Answer:
Verified
Q67: For the following reaction find Kp at
Q68: If Q increases,
A)ΔG increases and the reaction
Q70: Q79: When equilibrium is reached at constant temperature Q83: Consider the following gas-phase reaction of A2 Q87: The figure below represents the spontaneous reaction Q95: What is K if ΔG° = -18.0 Q96: Consider the reaction 2A(g)⇌ A2(g).The following pictures Q98: Calculate Ksp for PbI2 at 25°C based Q103: What is the entropy change associated with![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents