The figure represents the spontaneous evaporation of nitrogen in which liquid nitrogen,N2(l) ,becomes gaseous nitrogen,N2(g) : N2(l) → N2(g) .What are the signs (+ or -) of ΔH,ΔS,and ΔG for this process? 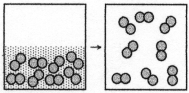
A) ΔH = +,ΔS = +,ΔG = +
B) ΔH = +,ΔS = +,ΔG = -
C) ΔH = -,ΔS = -,ΔG = +
D) ΔH = -,ΔS = -,ΔG = -
Correct Answer:
Verified
Q61: If ΔG° is negative for a reaction,
A)K
Q64: ΔG = ΔG° for a reaction
A)if Q
Q65: If ΔG is small and positive,
A)the forward
Q71: At high temperatures,boron carbide vaporizes according to
Q74: If ΔG° is positive for a reaction,
A)K
Q76: What is the relationship between ΔG,Qp,and Kp
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents