Calculate the equilibrium constant,K,at 25°C for the galvanic cell reaction shown below: 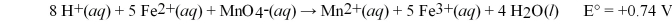
A) 3) 2 × 10-63
B) 3) 2 × 10-13
C) 3) 2 × 1012
D) 3) 2 × 1062
Correct Answer:
Verified
Q52: At 25°C,E° = +1.88 V for a
Q62: A cell based on the reaction below
Q63: The cell reaction for a lead storage
Q69: A particular 12V battery is based on
Q69: The equilibrium constant,K,can be calculated from
A)E°.
B)E.
C)either E°
Q70: Consider the following cell: Pt(s)∣ H2(g,p1)∣ H+(aq,pHA)∣∣
Q70: Given pH2 = 0.100 atm,[Cd2+] =
Q71: Given that E°red = -0.26 V for
Q76: For a particular cell based on the
Q77: The following cell has a potential of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents