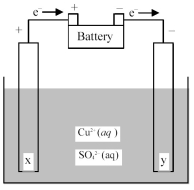
-Is the cell shown above a galvanic or an electrolytic cell? What is the direction of ion flow?
A) Electrolytic cell;Cu2+ ions flow toward electrode x and SO42- ions flow toward electrode y.
B) Electrolytic cell;Cu2+ ions flow toward electrode y and SO42- ions flow toward electrode x.
C) Galvanic cell;Cu2+ ions flow toward electrode x and SO42- ions flow toward electrode y.
D) Galvanic cell;Cu2+ ions flow toward electrode y and SO42- ions flow toward electrode x.
Correct Answer:
Verified
Q103: Shown below is a galvanic cell with
Q105: What is the shorthand notation that represents
Q110: Consider the following standard reduction potentials:
Ni2+(aq)+ 2
Q112: If the concentrations of Ag+(aq)and Cu2+(aq)are varied
Q124: Consider the following galvanic cell. 
Q129: Consider the galvanic cell shown below.
Q130: Shown below is an electrochemical cell with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents