Shown below is an electrochemical cell with anode a and cathode c.Both the anode and the cathode are inert electrodes.The liquid shown in compartment b of the cell is molten potassium chloride,KCl(l) . 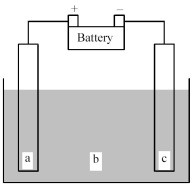
-Determine whether this is a galvanic or an electrolytic cell and give the reaction occurring at the anode and the reaction occurring at the cathode.
A) Electrolytic cell Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
B) Electrolytic cell Anode reaction: 2 Cl-(l) → Cl2(g) + 2e-
Cathode reaction: K+(l) + e- → K(s)
C) Galvanic cell Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
D) galvanic cell Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
Correct Answer:
Verified
Q81: How many grams of chromium metal are
Q99: Which of the following statements concerning the
Q101: Shown below is a galvanic cell with
Q113: Given the half-cell potentials below,calculate the cell
Q114: Identify the anode and cathode half-reactions and
Q120: The bright colors of anodized titanium are
Q123: The initial concentrations of Ag+(aq)and Cu2+(aq)are both
Q124: For a galvanic cell that uses the
Q125: Shown below is a galvanic cell
Q135: Consider the galvanic cell shown below.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents