What is the total volume of the mixture of hydrogen gas and oxygen gas can be obtained from the electrolysis of 90.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below?
2 H2O(l) 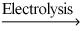 2 H2(g) + O2(g)
2 H2(g) + O2(g)
A) 61.1 L
B) 122 L
C) 183 L
D) 366 L
Correct Answer:
Verified
Q1: Which is not an isotope of hydrogen?
A)hydrogen-1
B)hydrogen-2
C)hydrogen-3
D)hydrogen-4
Q3: If the molar mass of monoatomic deuterium
Q5: The strongest homonuclear single bond is
A)H-H
B)C-C
C)N-N
D)O-O
Q6: What is not a commercial method for
Q7: How many grams of zinc metal are
Q8: Which is an ionic binary hydride?
A)HCl
B)KH
C)both HCl
Q9: How many liters of hydrogen gas are
Q10: How many kilojoules of energy are released
Q11: The dissociation of D2O is shown below.
D2O
Q14: Hydrogen,H2,has a very low boiling point.What is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents