In the following picture of an uncharged oxide,darkly-shaded spheres represent oxygen atoms or ions and lightly-shaded spheres represent atoms or ions of a second- or third-row element in its highest oxidation state.Identify the element represented by the lightly-shaded spheres. 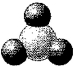
A) Al
B) C
C) N
D) S
Correct Answer:
Verified
Q100: When 0.500 grams of CuSO4 ∙ 5H2O
Q102: Look at the location of elements A,B,C,and
Q103: In the following picture of an oxide,darkly-shaded
Q104: In the picture representing binary hydride AHx,lightly-shaded
Q106: In the following picture of an oxide,darkly-shaded
Q107: In the following picture of an oxide,darkly-shaded
Q108: In the following picture of an oxide,darkly-shaded
Q109: In the following picture of an oxide,darkly-shaded
Q110: In the picture representing binary hydride AHx,lightly-shaded
Q164: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents