The reaction below releases ________ kJ/mol.The atomic masses are 3He (3.0160),1H (1.0078),4He (4.0026),0e (0.0005486). 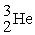
+ 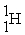
→ 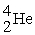
+ 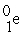
Correct Answer:
Verified
Q60: Which of the following isotopes is not
Q61: Energy is _ (released,required)in the transmutation reaction
Q62: What is needed to balance the nuclear
Q64: What is needed to balance the nuclear
Q68: What is needed to balance the nuclear
Q69: The mass change for the reaction shown
Q70: Carbon-14,which is present in all living tissue,radioactively
Q98: If the age of the Earth is
Q148: The binding energy for lithium-7 nuclei is
Q164: The mass defect for the chemical reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents