The mass spectrum of an element with two naturally occurring isotopes is shown below.Its average atomic mass would be best estimated as 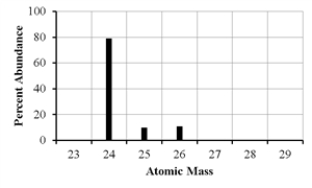
A) less than 26 amu but greater than 25 amu.
B) less than 25 amu but greater than 24 amu.
C) equal to 24 amu.
D) equal to 25 amu.
E) greater than 26 amu.
Correct Answer:
Verified
Q45: Neon has three naturally occuring isotopes.The abundance
Q46: In a particular mass of KAu(CN)2,there are
Q47: Choose the group containing the most reactive
Q48: Which element belongs to the alkali metals?
A)rubidium
B)germanium
C)barium
D)iodine
E)argon
Q49: The elements in groups 1A-8A or 1-2
Q51: The average atomic mass of Eu is
Q52: Which formula is best described as a
Q53: A periodic law based on atomic masses
Q54: A certain element is listed as having
Q55: Which of the following statements is not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents