An excess of sodium sulfate was added to a 1.000-L sample of polluted water.The mass of lead (II) sulfate that precipitated was 308.88 mg.Determine the mass of lead present in the sample of water.
Na2SO4(aq) + Pb(NO3) 2(aq) 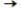
2NaNO3(aq) + PbSO4(s)
A) 108.15 mg
B) 88.02 mg
C) 180.08 mg
D) 145.56 mg
E) 211.03 mg
Correct Answer:
Verified
Q142: What volume of 1.08 M HCl is
Q143: In order to dilute 71.1 mL of
Q144: Identify the true relationship between molarity and
Q145: The reaction of HCl with NaOH is
Q146: In a volumetric analysis experiment,an acidic aqueous
Q148: A 20.00-mL sample of a weak base
Q149: An impure sample of benzoic acid (C6H5COOH,122.12
Q150: _ is a type of quantitative analysis
Q151: In a volumetric analysis experiment,a solution of
Q152: In order to determine the amount of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents