When the valve between the 2.00-L bulb,in which the gas pressure is 2.50 atm,and the 3.00-L bulb,in which the gas pressure is 1.50 atm,is opened,what will be the final pressure in the two bulbs? Assume the temperature remains constant. 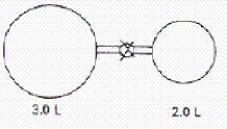
A) 1.90 atm
B) 4.00 atm
C) 2.17 atm
D) 2.10 atm
E) 1.83 atm
Correct Answer:
Verified
Q14: The following volume-temperature plots were made at
Q15: A sample of methane,CH4,occupies a volume of
Q16: The pressure of a certain gas is
Q17: A particular gas exerts a pressure of
Q18: A particular gas exerts a pressure of
Q20: A flexible vessel contains 37 L of
Q21: A flexible container is charged with a
Q22: What volume of sulfur trioxide gas,SO3,has the
Q23: What volume of gaseous oxygen,O2,has the same
Q24: A given mass of gas occupies a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents