A 1.00-L bulb contains a sample of O2 at 25°C and 1.00 atm pressure.A second 1.00-L bulb contains a sample of CH4 at 25°C and 1.00 atm pressure.What is the ratio of the number of molecules of methane to the number of molecules of oxygen in each of the containers?
A) 2:5
B) 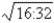
C) 5:2
D) 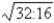
E) 1:1
Correct Answer:
Verified
Q25: A gas occupies a volume of 2.00
Q26: A gas occupies a volume of 2.75
Q27: Which of the following is a correct
Q28: A flexible container is charged with 79.00
Q29: A fixed amount of gas in a
Q31: A fixed amount of gas in a
Q32: The pressure of 6.2 L of nitrogen
Q33: A rigid container is charged with a
Q34: A gas occupying a volume of 1.50
Q35: Which of the following statements,concerning equal volumes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents