How much heat is evolved upon the complete oxidation of 6 g of aluminum at 25°C and 1 atm pressure? ( 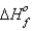 for Al2O3 is -1676 kJ/mol.)
for Al2O3 is -1676 kJ/mol.)
4Al(s) + 3O2(g) → 2Al2O3(s)
A) 9.238 × 103 kJ
B) 342.3 kJ
C) 684.7 kJ
D) 171.1 kJ
E) 85.59 kJ
Correct Answer:
Verified
Q21: What is the quantity of heat evolved
Q22: What is the change in enthalpy when
Q23: When 56.8 g of lead reacts with
Q24: Under conditions of constant pressure,for which of
Q25: Under conditions of constant pressure,for which of
Q27: Which of the following statements is incorrect
Q28: How much heat is liberated at constant
Q29: According to the following thermochemical equation,if 403.3
Q30: What quantity,in moles,of oxygen is consumed when
Q31: Consider the following thermochemical equation:
N2(g)+ 2O2(g)→ 2NO2(g);
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents