What is the energy of a photon of electromagnetic radiation with a wavelength of 963.5 nm? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A) 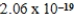 J
J
B) 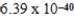 J
J
C) 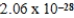 J
J
D) 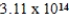 J
J
E) 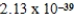 J
J
Correct Answer:
Verified
Q13: When a particular metal is illuminated with
Q14: What is the wavelength of a photon
Q15: What is the wavelength of a photon
Q16: A photon of red light has a
Q17: A light emitting diode (L.E.D.)emits photons with
Q19: Based on the photoelectric effect,Einstein proposed the
Q20: What is the wavelength of a photon
Q21: If the x-component of the velocity of
Q22: From the Bohr model of the hydrogen
Q23: What is the wavelength of light emitted
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents