Consider the following energy-level diagram for a particular electron in an atom. 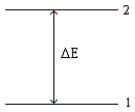 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?
A) The wavelength of a photon emitted by the electron jumping from level 2 to level 1 is given by 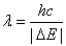 .
.
B) If the electron is in level 1,it may jump to level 2 by absorbing a photon with energy of ΔE.
C) If the electron is in level 1,it may jump to level 2 by absorbing any photon having energy of at least ΔE.
D) We would observe an electron jumping from level 2 to level 1 as a single line in a line spectrum.
E) If the electron is in level 2,it may jump to level 1 by emitting a photon with energy of |ΔE|.
Correct Answer:
Verified
Q24: What is the frequency of light emitted
Q25: A radial probability plot for an electron
Q26: Which of the following statements is a
Q27: What is the wavelength of an electron
Q28: When an electron in an atom makes
Q30: Whose postulates account for the line spectrum
Q31: Which of the following scientists first postulated
Q32: Who postulated that energy is radiated only
Q33: Which of the following statements is incorrect
Q34: The square of the wave function,ψ2,of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents