Which of the following sets of quantum numbers (n,l,ml, ms) refers to a 3d orbital?
A) 2 0 0 - 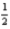
B) 5 4 1 - 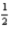
C) 4 2 -2 + 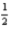
D) 4 3 1 - 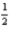
E) 3 2 1 - 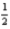
Correct Answer:
Verified
Q54: Which of the following combinations of quantum
Q55: Which of the following subshells does not
Q56: Which orbital or orbitals is/are specified by
Q57: How many p orbitals are in the
Q58: How many orbitals have the set of
Q60: The angular momentum quantum number is best
Q61: Which hydrogen atom orbital has an energy
Q62: The _ of a wave is the
Q63: What is the total number of subshells
Q64: Which of the following is a representation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents