What is the value of the spin quantum number for an electron in a 4d orbital?
A) 4
B) 2
C) either 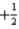 or
or 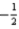
D) 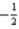
E) 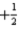
Correct Answer:
Verified
Q40: If the location of a particular electron
Q41: What is the value of the principal
Q42: How many values are there for the
Q43: An orbital with the quantum numbers n
Q44: Which of the following statements is incorrect?
A)The
Q46: All the following statements about the quantum
Q47: The number of orbitals having a given
Q48: Which quantum number distinguishes the different shapes
Q49: Which of the following sets of quantum
Q50: The number of orbitals in a p
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents