The Pauli exclusion principle states that
A) the wavelength of a photon of light times its frequency is equal to the speed of light.
B) no two electrons in the same atom can have the same set of four quantum numbers.
C) both the position of an electron and its momentum cannot be known simultaneously very accurately.
D) the wavelength and mass of a subatomic particle are related by 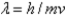 .
.
E) an electron can have either particle character or wave character.
Correct Answer:
Verified
Q3: Which of the following electron configurations corresponds
Q4: What is the maximum number of electrons
Q5: Which of the following statements is incorrect?
A)Stern
Q6: The maximum number of electrons that can
Q7: According to the building-up principle or aufbau
Q9: Which of the following orbital occupancy designations
Q10: Which element is found in the s-block
Q11: The ground-state valence-shell configuration of a particular
Q12: Which of the following electron configurations is
Q13: The ground-state valence-shell configuration of a particular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents