How many sigma and pi bonds are in the molecule pictured below? 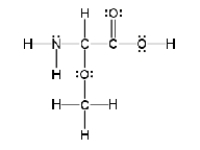
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
Correct Answer:
Verified
Q70: A π bond is the result of
Q71: What is the hybridization of I in
Q72: Which of the following molecules has a
Q73: When an atom in a molecule or
Q74: Which molecule or ion does not contain
Q76: Which of the following molecules is nonpolar?
A)SF4
B)PF5
C)ClF3
D)PF3
E)CH2F2
Q77: Which of the following statements is incorrect
Q78: Which of the following statements best describes
Q79: Which of the labeled carbons (C1-C4)is/are sp-hybridized?
Q80: According to valence-bond theory,the bonding in ketene,H2CCO,is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents