According to valence-bond theory,what is the hybridization scheme of the sulfur atom in SF4?
A) 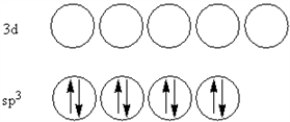
B) 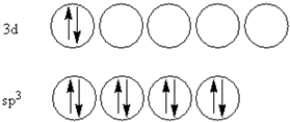
C) 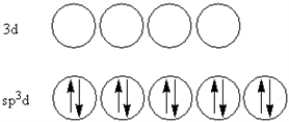
D) 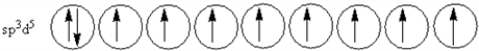
E) 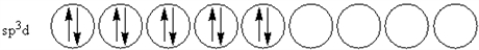
Correct Answer:
Verified
Q59: Which of the following characteristics does not
Q60: For which molecule or ion does the
Q61: What is the hybridization of Se in
Q62: What is the hybridization of Br in
Q63: Which one of the following statements provides
Q65: What is the hybridization of the nitrogen
Q66: What hybrid orbitals of sulfur are involved
Q67: Which of the following compounds is nonpolar?
A)H2S
B)XeF2
C)SO2
D)N2O
E)HCl
Q68: Which of the following molecules is polar?
A)SF6
B)CCl4
C)BF3
D)NO2
E)CO2
Q69: Which of the following statements is true?
A)A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents