Consider the following series of molecular ions and molecules: F2+,F22+,F2,and F2-.Which will have the shortest bond length between the fluorine atoms? Assume the homonuclear molecular orbital diagram provided below for nitrogen (excluding the K shells) still applies to these species. 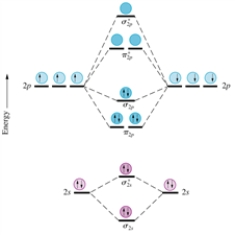
A) F2+
B) The bond lengths are all equivalent.
C) F22+
D) F2
E) F2-
Correct Answer:
Verified
Q91: Which molecule or ion has the shortest
Q92: Given the molecular orbital diagram for dinitrogen
Q93: If four orbitals on one atom overlap
Q94: Which of the following statements about the
Q95: Which of the following species has(have)a bond
Q96: The configuration (σ2s)2(σ2s*)2(π2py)1(π2px)1 is the molecular orbital
Q97: Which of these statements about benzene is
Q98: Which molecule or ion has the highest
Q100: Given the molecular orbital diagram for dinitrogen
Q101: The following statements concern molecules that require
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents