The enthalpy of fusion of aluminum is 10.7 kJ/mol.How many grams of aluminum can be melted by adding 81.4 kJ of energy to the metal at its melting point?
A) 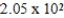 g
g
B) 32.2g
C) 3.01 g
D) 7.6 g
E) 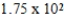 g
g
Correct Answer:
Verified
Q13: A bottle is filled with a small
Q14: If more ice is added to an
Q15: The vapor pressure of a liquid increases
Q16: What is the value of q when
Q17: Which of the following phase changes are
Q19: Which of the following statements concerning liquids
Q20: How much heat is released at constant
Q21: Which explanation best describes surface tension?
A)Molecules at
Q22: From a consideration of the phase diagram
Q23: The critical point of CCl4 is 283°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents