In the accompanying phase diagram,a liquid can be present at combinations of temperature and pressure corresponding to points 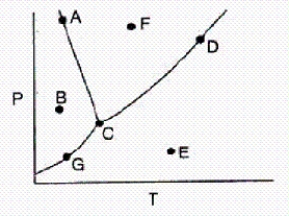

A) A,C,G,and D.
B) A,C,D,and F.
C) A,B,C,and G.
D) A and C only.
E) G,C,D,and E.
Correct Answer:
Verified
Q35: Knowing that ΔHvap for water is 40.7
Q36: For a particular liquid,raising its temperature from
Q37: Below is a phase diagram for a
Q38: Below is a phase diagram for a
Q39: Given the accompanying phase diagram,under what conditions
Q41: Which of the following indicates the existence
Q42: Which of the following pure substances has
Q43: Which compound has the lowest standard enthalpy
Q44: Which of the following pure substances has
Q45: In an experiment,40.0 mmol of helium gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents