Choose the correct statement about the diagram below. 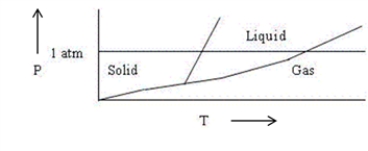
A) The diagram shows the triple point above 1 atm pressure.
B) The diagram is qualitatively correct for water.
C) The diagram shows that the melting point of the solid increases with increasing pressure.
D) The diagram could represent the phase diagram of CO2.
E) None of the above statements is correct.
Correct Answer:
Verified
Q20: How much heat is released at constant
Q21: Which explanation best describes surface tension?
A)Molecules at
Q22: From a consideration of the phase diagram
Q23: The critical point of CCl4 is 283°C
Q24: The triple point of iodine is at
Q26: In which of the following substances are
Q27: If the liquid of a pure substance
Q28: If the diameter of a spherical water
Q29: What is the enthalpy of vaporization of
Q30: Which of the following statements concerning the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents