The space-filling representation of a crystalline potassium provided below is an example of a _____ unit cell,which contains the equivalent of _____ atom(s) within a single unit cell.
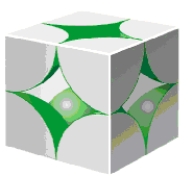
A) body centered cubic,2 atoms
B) face centered cubic,4 atoms
C) simple cubic,1 atom
D) body centered cubic,4 atoms
E) face centered cubic,5 atoms
Correct Answer:
Verified
Q83: How many atoms are there in a
Q84: In any cubic lattice an atom lying
Q85: Lithium chloride crystallizes in a face-centered cubic
Q86: Which of the following pure substances has
Q87: Which of the following pure substances has
Q89: How many atoms are there in a
Q90: Which of the following pure substances is
Q91: Which of the following pure substances has
Q92: For a given pure metal which of
Q93: A metal crystallizes in a face-centered cubic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents