Nickel crystallizes in a face-centered cubic lattice.The density of the element is 8.91 g/cm3.What is the volume of a single unit cell?
A) 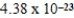 cm3
cm3
B) 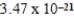 cm3
cm3
C) 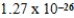 cm3
cm3
D) 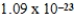 cm3
cm3
E) 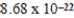 cm3
cm3
Correct Answer:
Verified
Q97: A low melting solid readily dissolves in
Q98: A solid crystal of NaCl is
A)soft,low melting,a
Q99: Which of the following pure substances has
Q100: Which one of the following statements about
Q101: The metal lead,with an atomic radius of
Q103: A metal crystallizes in a face-centered cubic
Q104: The metal sodium crystallizes in a body-centered
Q105: The metal lithium crystallizes in a body-centered
Q106: Calcium crystallizes in a face-centered cubic lattice.The
Q107: Platinum crystallizes with a face-centered cubic unit
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents