The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 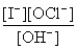
) If time is measured in seconds and concentration is measured in moles per liter,what are the units for k?
A) mol2/(L2 ∙ s)
B) L/(mol ∙ s)
C) 1/s
D) L2/(mol2 ∙ s)
E) mol/(L ∙ s)
Correct Answer:
Verified
Q8: Which of the following statements is always
Q9: Which of the following statements concerning the
Q10: For the reaction
6CH2O(aq)+ 4NH3(aq)→ (CH2)6N4(aq)+ 6H2O(l)
The rate
Q11: For which of the following hypothetical rate
Q12: Consider the reaction
AA + bB 
Q14: For the reaction
IO3-(aq)+ 5I-(aq)+ 6H+(aq)→ 3I2(aq)+ 3H2O(l)
The
Q15: For a certain first-order reaction with the
Q16: The oxidation of ammonia produces nitrogen and
Q17: Which of the following statements is true
Q18: For the reaction
2N2O5(g)→ 4NO2(g)+ O2(g)
Which of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents