The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 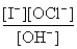
) The overall reaction order and the order with respect to OH- are
A) 2 and -1.
B) 0 and -1.
C) 0 and 1.
D) 2 and 1.
E) 1 and -1.
Correct Answer:
Verified
Q33: If a reaction is zero-order in a
Q34: The rate law for the hydrolysis of
Q35: The acid-catalyzed reaction of acetone,CH3COCH3,with iodine can
Q36: For the reaction between nitrogen monoxide and
Q37: The balanced chemical equation and rate law
Q39: The reaction between selenous acid and the
Q40: The rate law for the reaction between
Q41: The gas-phase decomposition of N2O5 is a
Q42: The reaction between selenous acid and the
Q43: A reaction that is second-order in one
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents