The gas-phase decomposition of N2O5 is a first-order process with a rate constant of 1.50 × 10-3 s-1 at 55°C.The decomposition reaction is
N2O5(g) → 2NO2(g) + 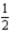
O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C) and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
A) Vessel 1 requires the same amount of time as vessel 2.
B) Vessel 1 requires twice as much time as vessel 2.
C) Vessel 1 requires three times as much time as vessel 2.
D) Vessel 1 requires four times as much time as vessel 2.
E) Vessel 2 requires twice as much time as vessel 1.
Correct Answer:
Verified
Q36: For the reaction between nitrogen monoxide and
Q37: The balanced chemical equation and rate law
Q38: The hypochlorite ion oxidizes the iodide ion
Q39: The reaction between selenous acid and the
Q40: The rate law for the reaction between
Q42: The reaction between selenous acid and the
Q43: A reaction that is second-order in one
Q44: For the hypothetical first-order reaction A →
Q45: For the hypothetical second-order reaction A →
Q46: A chemical reaction that is first-order in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents