The OH· radical disproportionates according to the elementary chemical reaction 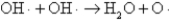 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
A) 2.8 × 101 s
B) 3.6 × 10-2 s
C) 1.8 × 10-2 s
D) 3.5 × 1011 s
E) 7.1 × 10-14 s
Correct Answer:
Verified
Q66: For the first-order reaction
1/2 N2O4(g)→ NO2(g); ΔH
Q67: Which of the following statements best describes
Q68: When the concentrations of the reactants are
Q69: The rates of most chemical reactions are
Q70: For a certain reaction of the general
Q72: For a certain reaction of the general
Q73: For the hypothetical reaction aA → products,the
Q74: The rate constant for a first-order reaction
Q75: For the hypothetical reaction A → products,the
Q76: In a first-order reaction,the half-life is 139
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents