For the hypothetical reaction A → products,the concentration of A was monitored over time.From the following graph,what is the rate constant for the decomposition of A? 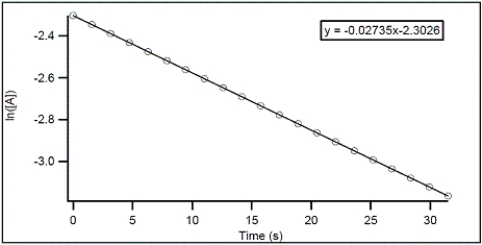
A) -0.02735 s-1
B) 2.3026 s-1
C) -2.3026 s-1
D) 0.02735 s-1
E) 0.01188 s-1
Correct Answer:
Verified
Q56: The reaction A → products is first-order
Q57: For which order reaction is the half-life
Q58: The nuclide 96Nb decays by a first-order
Q59: Dinitrogen tetroxide decomposes to form nitrogen dioxide
Q60: Which of the following corresponds to the
Q62: Which of the following corresponds to the
Q63: Which of the following is not a
Q64: For the following reaction producing 1 mol
Q65: The main reason for the increase in
Q66: For the first-order reaction
1/2 N2O4(g)→ NO2(g); ΔH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents