Given the following fast equilibrium (below) proposed for the gas phase reaction between NO and O2,what is the expression for the equilibrium concentration of the intermediate NO3?
NO + O2 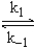
NO3
A) 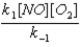
B) 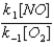
C) 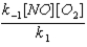
D) 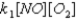
E) 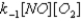
Correct Answer:
Verified
Q80: A first-order chemical reaction is observed to
Q81: The complete mechanism for a reaction is
Q82: One mechanism for the depletion of ozone
Q83: Which of the following statements is not
Q84: A mechanism that explains the rate law,Rate
Q86: Below is a proposed mechanism for the
Q87: Which of the following statements is incorrect?
A)The
Q88: The Arrhenius equation, Q89: The following mechanism has been suggested for Q90: The complete mechanism for a reaction is![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents