When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilbrium mixture of reactants and products according to the balanced chemical equilibrium below.
CO(g) + 3H2(g) 
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B? 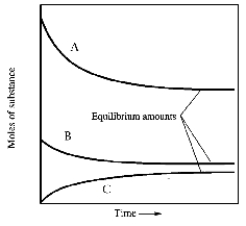
A) carbon monoxide
B) either methane or water
C) hydrogen
D) either hydrogen or carbon monoxide
E) not enough information to decide
Correct Answer:
Verified
Q12: Which of the following correctly describes the
Q13: A sample of ammonia gas was allowed
Q14: A 35.00-L vessel at 700 K initially
Q15: What is the balanced equation for the
Q16: What is the expression for Kc for
Q18: What balanced equation is the following equilibrium
Q19: Which expression correctly describes the equilibrium constant
Q20: Apply the law of mass action to
Q21: A sample of ammonia gas was allowed
Q22: Consider the following equilibrium:
1/2N2O4(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents