The reaction quotient for a system is 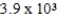 .If the equilibrium constant for the system is
.If the equilibrium constant for the system is 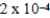
,what will happen as the reaction mixture approaches equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net gain in both product(s) and reactant(s) .
C) There will be a net gain in product(s) .
D) There will be a net gain in reactant(s) .
E) The equilibrium constant will decrease until it equals the reaction quotient.
Correct Answer:
Verified
Q44: For the equilibrium PCl5(g) Q45: Consider the following equilibrium: Q46: At 700 K,Kp for the following equilibrium Q47: In an experiment,0.30 mol H2 and 0.30 Q48: Consider the following equilibrium: Q50: At 800 K,Kc for the following equilibrium Q51: CS2(g)+ 3Cl2(g) Q52: Hydrogen iodide undergoes decomposition according to the Q53: Consider the following equilibrium: Q54: For the equilibrium N2O4(g) Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
4NH3(g)+ 3O2(g) 
2NO(g)+ 3F2(g) 
![]()
C2H6(g)+ C5H12(g) 
![]()

