Which of the following equilibria would not be affected by pressure changes at constant temperature?
A) CO2(g) + H2(g)  CO(g) + H2O(g)
CO(g) + H2O(g)
B) CO(g) + 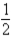 O2(g)
O2(g)  CO2(g)
CO2(g)
C) 2Hg(l) + O2(g)  2HgO(s)
2HgO(s)
D) 2H2(g) + O2(g)  2H2O(l)
2H2O(l)
E) CaCO3(s)  CaO(s) + CO2(g)
CaO(s) + CO2(g)
Correct Answer:
Verified
Q59: Consider the following equilibrium.
4NH3(g)+ 3O2(g) 
Q60: The equilibrium constant for the reaction H2(g)+
Q61: For which of the following systems at
Q62: At 550 K,Kp = 7.7 × 102
Q63: In which of the following reactions does
Q65: For which of the following systems at
Q66: Which of the following,when added to an
Q67: Consider the following equilibrium: Q68: Ammonia is prepared industrially by the reaction Q69: When cobalt chloride is added to pure
NH4Cl(s) ![]()
N2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents