An initially 1.0 M aqueous solution of a weak monoprotic acid has a total ion concentration of 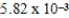 M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
A) 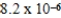
B) 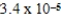
C) 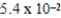
D) 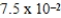
E) 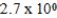
Correct Answer:
Verified
Q10: What is the equilibrium expression for the
Q11: What is the percent ionization of a
Q12: A 0.010 M aqueous solution of a
Q13: For which of the following equilibria does
Q14: A 0.10 M solution of a weak
Q16: At a temperature of 25°C an initally
Q17: What is the pH of an initially
Q18: Equal moles of the indicated acids are
Q19: A 0.10 M solution of a weak
Q20: A 0.20 M solution of a weak
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents