What is the equilibrium percent ionization of an initially 0.32 M solution of the monoprotic acid valeric acid,HC5H9O2,at 25°C (Ka = 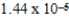 ) ?
) ?
A) 0.67%
B) 1.30%
C) 0.0014%
D) 0.1%
E) 2.00%
Correct Answer:
Verified
Q18: Equal moles of the indicated acids are
Q19: A 0.10 M solution of a weak
Q20: A 0.20 M solution of a weak
Q21: What is the equilibrium hydronium ion concentration
Q22: What is the equilibrium pH of an
Q24: In a 0.20 M solution of a
Q25: What is Ka for a weak monoprotic
Q26: In a 0.01 M solution of 1,4-butanedicarboxylic
Q27: What is the pH of a solution
Q28: In a 0.01 M solution of 1,4-butanedicarboxylic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents