What is the equilibrium pH of an initially 4.4 M solution of hypochlorous acid,HOCl,at 25°C (Ka = 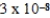 ) ?
) ?
A) 3.44
B) 7.37
C) 10.41
D) 10.71
E) 4.08
Correct Answer:
Verified
Q24: In a 0.20 M solution of a
Q25: What is Ka for a weak monoprotic
Q26: In a 0.01 M solution of 1,4-butanedicarboxylic
Q27: What is the pH of a solution
Q28: In a 0.01 M solution of 1,4-butanedicarboxylic
Q30: Carbonic acid,H2CO3,is a weak diprotic acid.In a
Q31: Phosphoric acid,H3PO4,will undergo three successive ionization reactions
Q32: For a 0.05 M H2SO3 solution,which of
Q33: A 8.42-g sample of homogentisic acid,a weak
Q34: What is the equilibrium concentration of chloroacetic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents