The amphiprotic anion monohydrogenphosphate acts as both an acid and a base in aqueous solution.Given the following acid-ionization and base-hydrolysis constants,what will be the approximate equilibrium pH of an aqueous solution of sodium monohydrogenphosphate?
Ka = 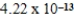
Kb = 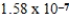
A) basic
B) acidic
C) neutral
Correct Answer:
Verified
Q70: Which of the following statements is true
Q71: Which of the following solutions has the
Q72: Given the following,what will be the approximate
Q73: Given the following,what will be the approximate
Q74: Which of the following salts will produce
Q76: A 0.0678 M solution of a weak
Q77: What is Ka for the anilinium cation,C6H5NH3+,at
Q78: Which of the following salts forms an
Q79: Which of the following salts will produce
Q80: Which of the following equilibria best represents
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents