What is the equilibrium fluoride ion concentration of a solution that initially consists of 0.23 M HF and 0.400 M HCl? Ka for HF is 6.8 × 10-4.
A) 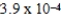 M
M
B)  M
M
C) 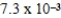 M
M
D) 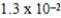 M
M
E) 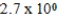 M
M
Correct Answer:
Verified
Q91: What is the pH of a solution
Q92: What is the pH of the solution
Q93: What is the pOH of a solution
Q94: Calculate the pH of a solution that
Q95: What is the hydroxide-ion concentration in a
Q97: For a solution equimolar in HCN and
Q98: What is the pH of a 0.24
Q99: The two acid-ionization constants for sulfurous acid,H2SO3,are
Q100: What is the pH of a solution
Q101: Which of the following will give a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents