Which acid-base combination is depicted by this titration curve? 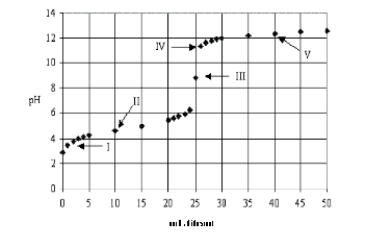
A) Titration of a weak base with a strong acid.
B) Titration of a weak acid with a strong base.
C) Titration of a strong acid with a strong base.
D) Titration of a strong base with a strong acid.
E) Not enough information provided.
Correct Answer:
Verified
Q128: A 75.0-mL sample of 0.0500 M HCN
Q129: A 25.00-mL sample of propionic acid,HC3H5O2,of unknown
Q130: The following titration curve depicts the titration
Q131: A solution contains 10.mmol of H3PO4 and
Q132: A weak base is titrated with a
Q134: Titration of 0.2834 g of an unknown
Q135: Which of the following statements is true
Q136: A 25.00-mL sample of propionic acid,HC3H5O2,of unknown
Q137: A sample of ammonia (Kb = 1.8
Q138: What is the hydronium-ion concentration of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents