Figures I-IV represent ionic compounds formed upon the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A.Identify the figure(s) that represent(s) products for which Ksp = s2,where s is the molar solubility of the ionic compound. 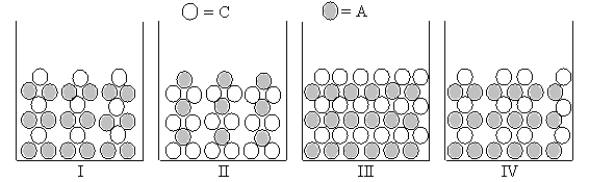
A) only I
B) only II
C) only IV
D) only III
E) both I and II
Correct Answer:
Verified
Q7: What is the solubility product expression for
Q8: After mixing an excess PbCl2 with a
Q9: The solubility of calcium carbonate in water
Q10: The solubility of lead(II)sulfate is 4.0 ×
Q11: What is the solubility product expression for
Q13: What is the relationship between molar solubility
Q14: What is the solubility product expression for
Q15: The solubility of silver(I)carbonate is 3.6 ×
Q16: What is the solubility product expression for
Q17: Cation C and anion A form an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents