The solubility of silver(I) carbonate is 3.6 × 10-2 g/L.What is the solubility product constant for silver(I) carbonate?
A) 4.4 × 10-15
B) 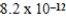
C) 1.7 × 10-8
D) 1.3 × 10-4
E) 1.3 × 10-3
Correct Answer:
Verified
Q10: The solubility of lead(II)sulfate is 4.0 ×
Q11: What is the solubility product expression for
Q12: Figures I-IV represent ionic compounds formed upon
Q13: What is the relationship between molar solubility
Q14: What is the solubility product expression for
Q16: What is the solubility product expression for
Q17: Cation C and anion A form an
Q18: Figures I-IV represent ionic compounds formed upon
Q19: What is the solubility product expression for
Q20: Which of the following particulate views is/are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents