Which Figures I-IV represent(s) the result of mixing aqueous solutions of NaOH and CuCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation,A = anion) 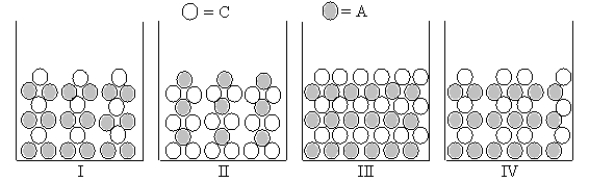
A) only II
B) both I and II
C) only IV
D) only I
E) only III
Correct Answer:
Verified
Q50: Ksp for PbF2 is 4.0 ×10-8.If a
Q51: What is the hydroxide-ion concentration of a
Q52: Suppose 50.00 mL of 2.0 × 10-6
Q53: What is the molar solubility of MgF2
Q54: Which of Figures I-IV represent(s)the result of
Q56: The figure below represents the result of
Q57: In which of these solutions would silver(I)carbonate
Q58: Which salt has the lowest molar solubility
Q59: In which of the following solutions would
Q60: What is the molar solubility of MgF2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents